What Is The Purpose Of The Periodic Table Quizlet
It defines periods and groups and describes how various electron configurations affect the properties of the atom. Members of a group typically have similar properties and electron configurations in their outer shell.

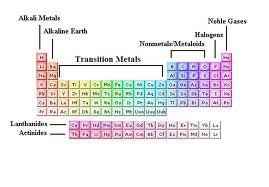
Areas On The Periodic Table Diagram Quizlet
Pour it down the sink.

What is the purpose of the periodic table quizlet. The periodic table is a tabular arrangement of the chemical elements. Based on the properties that were displayed by the known elements Mendeleev was able to predict where there were holes in his table or elements yet to be discovered. The elements along side the line are metalloids.
If youre reading this you better smile cause you arent the only one stressed out about school have a great day love. The modern periodic table is based on Dmitri Mendeleevs 1896 observations that chemical elements can be grouped according to chemical properties they exhibit. The electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or.
Test your bond with the periodic table of elements in this quiz on all 118 chemical elements and their symbols. The periodic table was constructed in 1869 by Dmitri Mendeleev. Chemical Bonding Covalent Bonds.
Laminiaduo7 and 218 more users found this answer helpful. They have relatively high electronegativities. The SEO Periodic Table just helps you to understand the factors and their impact on SEO.
Classification elements into groups with similar properties. To predict the possibilities of new elements based on their properties. Before all the naturally occurring elements were discovered the periodic table was used to predict the chemical and physical properties of elements in the gaps on the table.
Before all naturally occurring elements were discovered the periodic table was used to predict the chemical and physical properties of elements in the gaps on the table. Period A horizontal row in the periodic table. The main purpose of the periodic table was.
You may be familiar with the chemical symbols for hydrogen and oxygen but can you match such lower-profile elements as gadolinium and erbium with their corresponding symbols. This module explains the arrangement of elements in the period table. Introduction to Chemical Bonds.
The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers electron configurations and chemical properties. It is organized in order of increasing atomic number. The modern periodic table is very similar to Mendeleevs table but elements today are ordered by increasing atomic number which reflects the number of protons in an atom.
With so many factors involved in SEO you have to do a lot of research and be up to date with the strategies you use. The periodic table is a central tool in helping us to understand regularities in the behavior of elements and compounds. Mendeleev developed the Periodic Table in 1869.
In the nineteenth century people noted similarities among various elements and tried to find a pattern of relationship among them. Today the table can be used to predict properties of elements yet to be discovered although these new elements are all highly radioactive and break down into more familiar. There is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties.
How is the purpose of the zigzag line in the periodic table. Today the table can be used to predict properties of elements yet to be discovered although these new elements are all highly radioactive and break down into more familiar elements almost instantly. The atomic number of each element increases by one reading from left to right.
A vertical column in the periodic table. Silicon for example is a semiconductor. The periodic table was built to show the relationships among the various elements.
So the elements can exercize on the stairs instead of just a flat surface What is to the right of the zigzag line on the periodic table. They have both metallic and nonmetallic character. Elements are placed on the periodic table based on their atomic structure.
From there its your decision to use or not to use certain factors for SEO. He arranged 63 elements into groups and left gaps in the table because he predicted new elements would be. The elements on the immediate right of the line tend to gain electrons more than lose them.
C increases from left to right across the periodic table.

Periodic Table The Periodic Table Flashcards Quizlet

General Chemistry Periodic Table Of Elements Diagram Quizlet

Periodic Table Diagram Quizlet

Periodic Trends Diagram Quizlet

Periodic Table Arrangement Flashcards Quizlet

Periodic Table Review Flashcards Quizlet

A Level Chemistry Ocr Mod 2 Chap 2 Flashcards Quizlet

Understanding The Periodic Table Diagram Quizlet

Periodic Table Periods And Groups Flashcards Quizlet

Periodic Table Mnemonic Story Flashcards Quizlet

The Periodic Table Of Elements Flashcards Quizlet

Kaplan Mcat General Chemistry Chapter 2 Periodic Table Diagram Quizlet

The Periodic Table Flashcards Quizlet

Pearson Science Topic 1 Atoms And The Periodic Table Diagram Quizlet

Chemistry 1 Honors The Periodic Table Diagram Quizlet

Periodic Trends Final Complete Flashcards Quizlet


Posting Komentar untuk "What Is The Purpose Of The Periodic Table Quizlet"